
Galvanocoagulation ecological sal method of current sewage treatment from ions of heavy metals.
 Alexey Vasilenko 1,
Alexey Vasilenko 1, Lesya Vasilenko 2*
Lesya Vasilenko 2*
1,2Kyiv National University of Construction and Architecture, Vozduhoflotsky Avenue 31, Kyiv, 03680, Ukraine
Received: 10/20/2017, Accepted: 12/10/2017, Available online: 12/28/2017.
DOI: https://doi.org/10.32557/useful-1-2-2017-0004
HDL: http://hdl.handle.net/20.500.12334/36
*Corresponding author: e-mail: e.voloshki@gmail.com
Under a creative commons license. Volume 1, Issue 2, 2017, pages: 30-35.
Author Keywords: wastewater, heavy metal ions, galvanocoagulation, filter loading
Abstract.
It implemented a general description of the washing waste water in electroplating. The analysis of existing methods of cleaning the wash wastewater containing heavy metal ions. The comparative characteristic and disadvantages of these methods are given. The method proposed galvanocoagulation engineering calculation of industrial filter for wastewater treatment from heavy metal ions, in which the galvanocoagulation process.
Introduction
Wastewater of galvanic industry is about from 30% to 50% of total amount of wastepaper that is formed at machine-building enterprises. In Ukraine and abroad electroplating industry wastewater is the most widespread one. Industrial wastewater polluted by acids, alkalis and salt of heavy metals is formed as a result of chemical and electrochemical processing of metals and their alloys as well as because of galvanic coating.
Galvanic industries are exploited in Ukraine at about 4000 enterprises of mechanical engineering, tool engineering, metal processing, ferrous and nonferrous metallurgy and other sectors of industries. Total amount of wastewater discharge by the above-mentioned industries in Ukraine is about 500 mln. m3annually.
Heavy metals and aluminum, cooper, nickel, zinc in wastewater from galvanic production are in ionic condition [1, 2] and according to the suggested classification of water purification they use the following principles:
- transferring to slight dissociated (neutralization, complexing) or low-soluble entities (formation of salts and hydrates);
- fixation on solid phase of ion-exchangers, separation by water physical state changing (distilling, freezing out);
- redistribution of ions in liquid phase (extraction, reverse osmosis) and ions mobility in electric and magnetic fields;
Engineering tasks solving while developing technological schemes, methods of extraction of ions of heavy metals from water are specified according to the forces of influence on impurities [3, 4, 5]:
- chemical methods;
- physical and chemical methods;
- physical methods.
Chemical methods are based on influence on impurities of volume forces of ionic and ionic-molecular impact which elicit structural and chemical changes in water system (reagentizing, oxidizing, processing with complexing agents, depositions and neutralizing agents, anion exchange).
Physico-chemical methods are based on effecting on water system by external physical force fields (acoustic, electric, electromagnetic, thermal) which cause structural and chemical changes in the system (electrocoagulation, electrophoresis, magnetic and ultrasound processing, freezing and other).
Physical methods are based on effects on the water system of physical forces (the pressure, thermal effect) or inner surface molecular forces which will lead to remove the impurities without structural and chemical changes in the system (hyperfiltration, vacuum detachment, coagulation, flocculation, adsorption, thermal detachment).
According to the method of purification processing we may combine them into the following groups: reactant, sportive, electrochemical, ion-exchange.
While conducting the research of wastewater purification along with the mentioned above methods there are other processes and phenomena which can change the effectiveness of the purification. For example, during the reactant purification the processes of coagulation appear, and ion-exchange is accompanied by the process of adsorption, at the same time on peculiar conditions some mineral sorbents discover the properties of ion exchangers etc.
Presentation of the main material of the study
Analysis of literary data has shown that the main widespread methods of galvanic industry wastewater purification from ions of heavy metals are reactant and electrocoagulation ones. However, the technological decisions used nowadays need a lot of reagents or energy carriers; also, they do not allow to create a compact block of treatment plants and to intensify the process considerably.
The most contemporary method of washing wastewater purification from heavy metal compounds and the most economic one is galvanic coagulation [1,2].
The method of galvanic coagulation allows to abandon the usage of reagents and to dramatically reduce the number of treatment plants due to the formation of ferrous oxide forms which leads to faster precipitation and allows to take away deficient components - plate steel (that is common for the electric coagulation method).
The method of galvanic coagulation [1,2] purification of wastewater from ions of heavy metals is based on water treatment in the field of multiple short-circuited galvanic couples.
This method is based on appearing of short-circuited galvanic couple between the elements bearing different electrochemical potential. Galvanic coagulation has already been used at some enterprises. The suggested scale in which galvanic coagulation process will appear consists of the materials that are mainly used at the enterprises. These are the grains of absorbent coal that act as one of the elements of galvanic couple in the process of galvanic coagulation and metal.
The mechanism of the process of galvanic coagulation takes place during the contact of contaminated wastewater with the filling. This process arises basing on the phenomena of interaction of substances with different electrochemical potentials in electrically conducting media.
Substances create the short-circuited galvanic couple in which the substance with smaller electrochemical potential to another substance acts as anode, and the second one - is catelectrode.
Let us consider the chemistry of the process of galvanic coagulation. The water stream, which is needed to be eliminated, is passed through the filter which consists of parametric catelectrode (activated carbon) with magnesium anode. While the water is passing the parametric filling the particles of anode substance (magnesium) collide with the surface of activated carbon and discharge on it, electrochemical de-electronation of magnesium takes place.
The process of galvanic circle can be presented as the following scheme:
Anode - electrolyte - catelectrode, i.e. Mg2+ - solution Me+ - activated carbon.
It’s crucial advantages before the traditional reagent method are obvious: significant reduction or complete refusal from chemical reagents usage; significant decrease of salt content and of hardness of the processed water; imperceptible electricity consumption; good water return.
The results of experimental researches which are given in the surveys of the author [4, 5] allow us to recommend the filtration through modified fillers for realization of purification process. In the suggested filter the anode is a magnesium rod. The standard electronic potential of magnesium is E=-2,37 B that is lower than the electronic potential of activated carbon E= -0,03 B, that causes the creation of a galvanic couple.
Let us give the methodology of such filters estimation.
For definition of necessary parameters of a filter (flat area, cubage), the quantity of magnesium rods, their mass and their diameter we should do the following: consider the height of a filtration layer as Hcp= 1,5m, the speed of filtration vcp=5 m/h, contact period tcont=18 min. (0,3 hours) [1, 2].
We estimate the flat area of filter
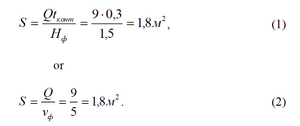
Cubage of magnesium rods before the filling to 1 m3it must be 0,007 (that is determinated from experimental researches). The length of a rod in relation to the height of filler is accepted as

Considering the mentioned above we may define the cubage of rods for the filter.
So, the cubage of filter filling will be
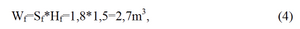
and the cubage of magnesium rods
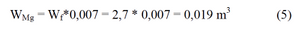
We accept the length of rod equal
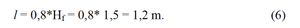
To define the number of rods and their diameter you need to consider the proportions of the filter multiple of 0,3 m - that is the largest distance between the rods, which is defined experimentally. if the filter is round shaped its diameter will be
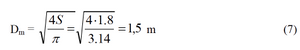
Thus, considering the diameter there will be 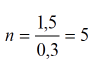 rods in the circle which is less than 0,5m of the filter frame, i.e. d=1.2m there must be
rods in the circle which is less than 0,5m of the filter frame, i.e. d=1.2m there must be ![]() - we accept 12 pcs, and if the diameter d=1,5 - 6 pcs.
- we accept 12 pcs, and if the diameter d=1,5 - 6 pcs.
If the rectangular shape of filter is used 1,5 1,5 the number of rods will be 19 pcs. When the number of rods is 19 pcs and their length is 1,2m - the diameter must be
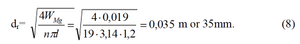
We can also define the mass of magnesium rods per one filter which will be
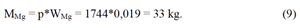
During the experiments, the ratio of the flat area of magnesium rods to the flat area of filter was

In the calculated rectangular filter
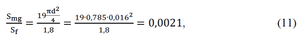
In the round shaped filter

Thus, we can calculate the industrial filter in which galvanic coagulation process takes place in order to purify washing wastewater from ions of heavy metals.
Conclusion
The given method shows that it is possible to use pressure filters, which need much cheaper reconstruction, in order to make a process of washing wastewater purification from ions of heavy metals. The filter is filled with activated carbon that has been already used at water treatment station with granules size of 0,5 - 0,6mm to the height h = 1,5m, also magnesium rods are placed into the filter, their length 0,8h and diameter is calculated. The effect of water purification is 98-99%. Because of carbon being used repeatedly the ecological effect is obvious.
References
[1] Riazantsev A.A., Batayev V.B., Timurova L.V., "Galvanic and coagulation purification of wastewater" Chemistry for sustainable development. - 1996. - 4 №3 - P. 233-241. rus.
[2] Riazantsev A.A., Batayeva A.A. "Purification of wastewater of additional industry from ions of heavy metals at the railroad enterprises" Problems of hydraulics, water supply, sewage. Collection of scientific works. Syberia national university of means of communication. Novosibirsk: Publishing house SNUMC. 2001. P. 54 - 66.
[3] Sokolova L.P., Kokorina E.B., Smurova E.S. "Galvanic coagulation purification of wastewater at the non-ferrous metal processing plant". Non-ferrous metallurgy 1990 №9 P. 56 - 60.
[4] Feofanov V.A., Zhdanovich A.P., Luhanin B.S. "Galvanic coagulation purification of wastewater", Alma-Ata. 1987, 70 p.
[5] Vasilenko L.A. Purification of Chromium containing wastewater "City municipal economy". scien. - techn. collection. #14, Kiev "Technika" 1998. P. 69 - 71.
[6] Vasilenko L.A. Purification of wastewater from chromium "City municipal economy". scien. - techn. collection #15. - Kiev.: Technika, 1998. P 56-57.
Please cite as: A.Vasilenko, L.Vasilenko “Galvanocoagulation ecological sal method of current sewage treatment from ions of heavy metals.” USEFUL online journal, vol. 1, no. 2, pp. 30–35, December 2017. DOI: https://doi.org/10.32557/useful-1-2-2017-0004







Comments